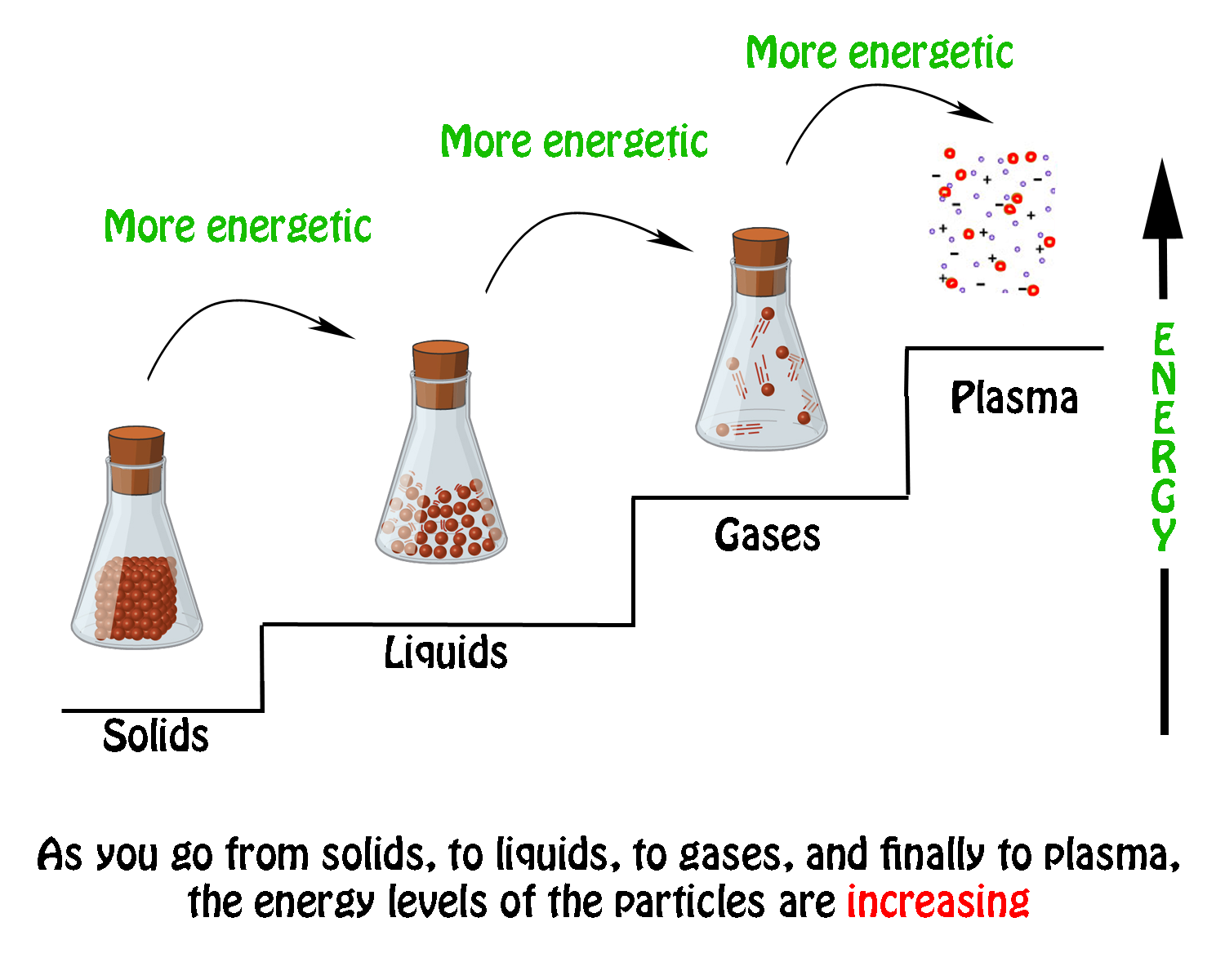
What Phase Changes Are Exothermic. When a substance loses heat energy the attractive forces between atoms slow them down reducing their mobility. All phase changes are accompanied by changes in the energy of a system. Fest flüssig und gasförmig. In the same manner at room temperature heat.

What are the endothermic phase changes. Exothermic An exothermic phase change releases heat energy into its environment. When a substance loses heat energy the attractive forces between atoms slow them down reducing their mobility. Freezing when a liquid transforms to solid. When temperature or pressure increases molecules interact more with each other. All phase changes are accompanied by changes.
What are the endothermic phase changes.
The three states of matter are solid liquid and gas. As time passes and water temperature drops the gas molecules water vapor around that glass slow down and change state from gas to liquid as they collect on the surface of the glass. Phase changes typically occur when the temperature or pressure of a system is altered. 1 -2 class periods. The following are exothermic phase changes. Also Know which change is exothermic.