Water Molecule Definition Biology. As you probably already know a water molecule consists of two hydrogen atoms bonded with one oxygen atom. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. Because it is a polar covalent molecule it. As you may already know its molecular formula is H2O.
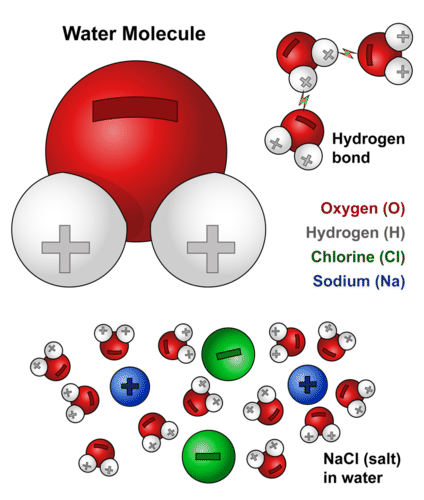
As you probably already know a water molecule consists of two hydrogen atoms bonded with one oxygen atom. Thus droplets or beads of water form on a nonpolar surface because water molecules adhere together instead of adhering to the surface. A molecule of water is made up of one oxygen atom to which two hydrogen atoms are attached via covalent bonds making a bond angle of 1045 o. The positive charge comes from the atomic nucleus while the electrons supply the negative charge. As you may already know its molecular formula is H2O. Such examples of charged polar molecules such as salts amino acids and sugars are able to readily dissolved in water.
Water molecules are the driving force of osmosis.
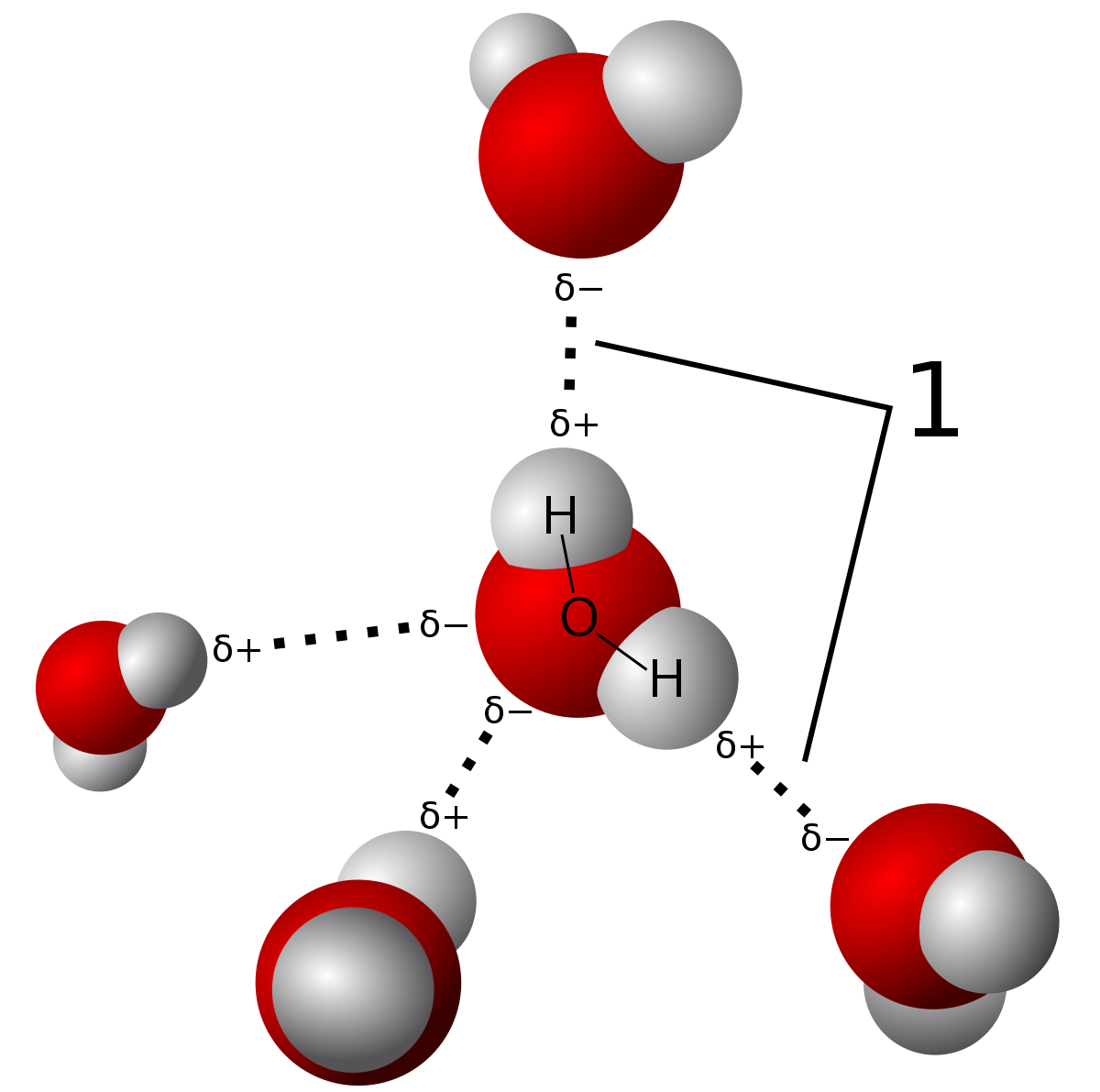
All water molecules are held together via strong hydrogen bonds among them. The oxygen atom in a water molecule is highly electronegative which means that it will pull the electrons in a bond closer to it. Not only does water exist in nature in all three states of matter solid liquid gas it also covers 75 percent of the earth and composes roughly 78 percent of the human body. Hydrophilic Molecules or substances that are attracted to water. As you probably already know a water molecule consists of two hydrogen atoms bonded with one oxygen atom. Such examples of charged polar molecules such as salts amino acids and sugars are able to readily dissolved in water.