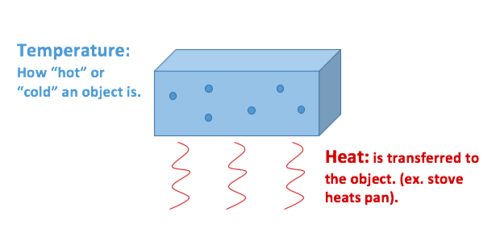
Similarities Between Thermal Energy And Temperature. The main difference is that heat deals with thermal energy while temperature is more concerned with molecular kinetic energy. The temperature of the hot body falls. The cold body gains energy and its temperature rises. So we can say that heat is afunction of Temperatureenergy stored in molecules.
Thermal energy is energy. Thermal energy can be otherwise understood as the total microscopic kinetic and potential energy of a system. Thats the energy that makes molecules in a fluid move randomly. The iron is then quickly transferred to water held in a thermally insulated container. Temperature describes the average kinetic energy of molecules. Temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy transferred between two materials that are at different temperatures.
Temperature is a parameter which can be directly measured with the help of various devices such as thermometers whereas thermal energy can be calculated.
The core differenceis that heat deals with thermal energy whereas temperatureis more concerned with molecular kinetic energy. Thermal energy can be otherwise understood as the total microscopic kinetic and potential energy of a system. The iron is then quickly transferred to water held in a thermally insulated container. Temperature describes the average kinetic energy of molecules. In classical mechanics temperature of a body indicates the average kinetic energy of all the molecules of the corresponding body. Then how is temperature different from thermal energy.