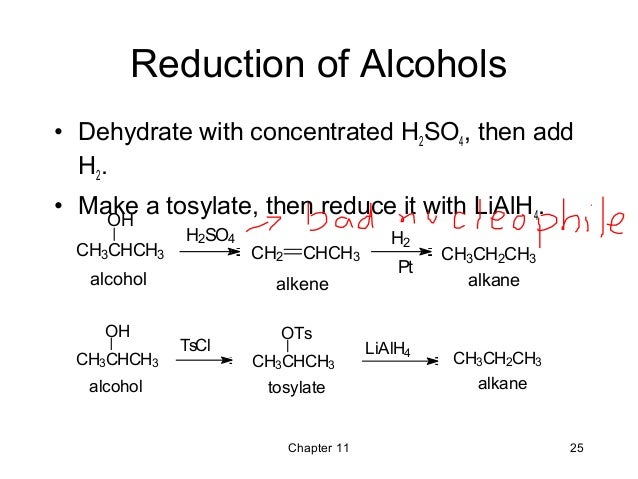
Reduction Of Alcohols To Alkanes. Synthetic Communications 1996 26 1 101-111. It combines with iodine forming phosphorus tri-iodide PI 3. You may have many products from halogenation. I have been looking everywhere for hours now and cant find anything on a simple lab reduction of an alcohol to an alkane that doesnt involve any fancy reagents such as tosylates metal catalysts etc.

Brønsted acids may also be used although cationic skeletal rearrangements and nucleophilic attack of the conjugate base on the carbocation may be problematic. Therefore it makes the reaction to. For reductions of aldehydes and ketones an aluminium hydride ion reduces the compound to form an alkoxide salt. Discussion of how to transform alcohols into alkenes using a two step reaction series. Synthetic Communications 1996 26 1 101-111. La Loggia James W.
Halogenation shuld be done either at high temperature or under light.
Water and a weak base. For reductions of aldehydes and ketones an aluminium hydride ion reduces the compound to form an alkoxide salt. The purpose of red phosphorus is to remove iodine. In this Reaction we shall see how to prepare Alkanes from Alcohols or how we may reduce Alcohols to Alkanes. For reductions of carboxylic acid derivatives after reduction by an aluminium hydride ion an elimination leads to the aldehyde product which can be reduced a second time to an alcohol. Benzylic alcohols secondary alcohols and tertiary alcohols were effectively reduced to give the corresponding alkanes in high yields.