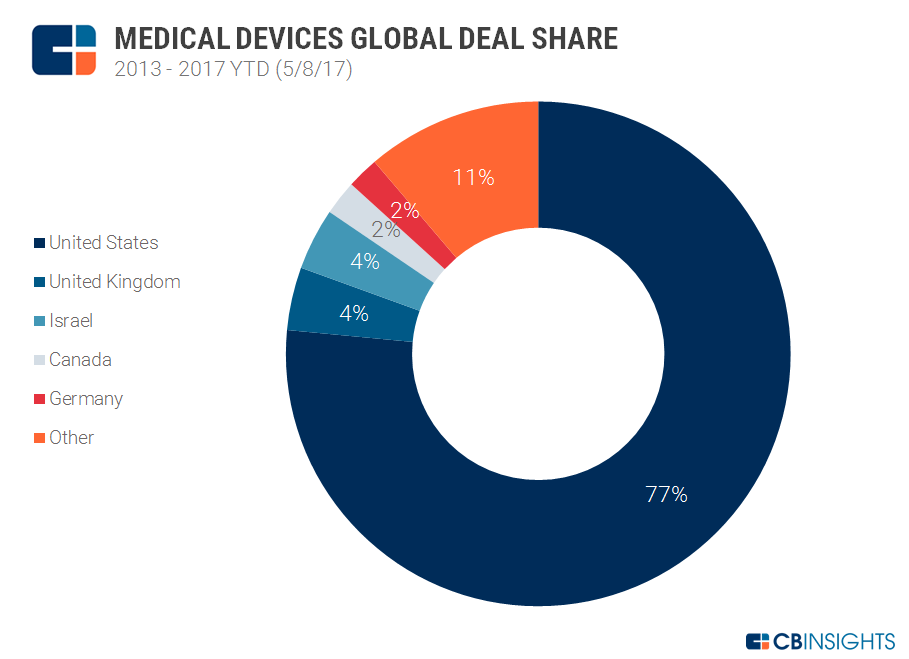
Medical Device Startups 2017. Elsa lets patients become their own personal researchers by providing them with a better understanding around how lifestyle factors may affect their disease activity measurements for. Die Verordnung EU 2017745 über Medizinprodukte ist am 25. Besides extended reality modes like VR AR and mixed reality have also been transforming the medica device industry. Sie gilt in den Mitgliedstaaten der Europäischen Union unmittelbar und muss daher nicht in nationales Recht umgesetzt werden.
Funding is always a major challenge for a startup. Top 3 Challenges for Medical Device Startups Challenge 1. In order for manufacturers to be able to demonstrate the confor mity of such products the. Funding and Capital Issues. Let us help you you will have a smooth transition to the new regulation. JADE is developing its first wearable healthcare technology device the JADE Model 01 final Beta product name yet to be announced Cloud-computed Radial Arterial Pulse Monitor.
Definition of a medical device or are covered by this Regulation.
12 Cer tain groups of products for which a manufacturer claims only an aesthetic or another non-medical pur pose but which are similar to medical devices in terms of functioning and r isks profile should be covered by this Regulation. For medical device startups theres the added burden of operating in a highly-regulated industry. Founded in 2013 the startup went on to raise 5 million Series A in March 2019. NeuSpera Medical is committed to bringing forward implantable medical device technology that will improve lives of patients battling with chronic illness. DxtER developed by Basil Leaf Technologies under the Final Frontier Medical Devices team name is a tricorder prototype that uses an artificial intelligencebased engine that. According to a survey done by Emergo half of medical device companies say that they face funding challenges.