How To Find Extinction Coefficient. Use the formula A ebc where A is absorbance e is molar extinction coefficient b is path length cm and c is concentration molL. This video explains how to work out the extinction coefficient for a solution from a st. Protein Extinction Coefficients and Concentration Calculation. In this equation e is the molar extinction coefficient.

Finally calculate the extinction coefficient. Using the Beer-Lambert Law we say that. 6 A log 10 I o I ϵ l c The constant ϵ is called molar absorptivity or molar extinction coefficient and is a measure of the probability of the electronic transition. L is the path length of the cell holder. Indeed the slope of your absorption spectrum would be your extinction coefficient as long as your pathlength is fixed according to Beer-Lambert law and you can accurately determine the. Using this we can now determine how much the 1mag kpc impacts.
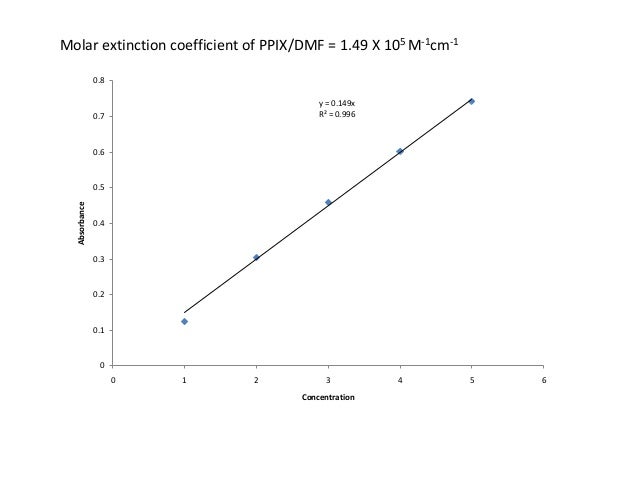
C is the concentration of the solution.
6 A log 10 I o I ϵ l c The constant ϵ is called molar absorptivity or molar extinction coefficient and is a measure of the probability of the electronic transition. This video explains how to work out the extinction coefficient for a solution from a st. Do this by measuring the absorbance of the provided solution or several dilutions thereof ideally prepared in duplicate or triplicate and then applying the formula A c L ε. In reality molar absorptivity constant is normally not given. Calculating the extinction coefficient - YouTube. Finally calculate the extinction coefficient.