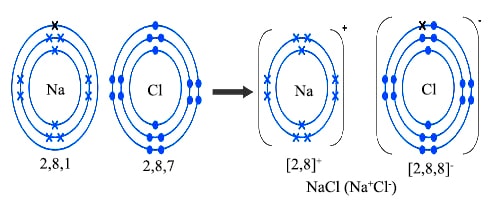
How Sodium Chloride Is Formed. Salt such as sodium chloride is formed when an acid and a base are neutralized in a chemical reaction. Characteristics of a Sodium Chloride Sodium chloride is formed when sodium atoms interact with chlorine atoms. Hence the salts get dissolved then the solution is pumped out. Sodium has a configuration of 281.

Sodium chloride crystal is made up of sodium and chloride ions. Ever get a taste of seawater in your mouth at the beach These deposits are mined for various salts including enough sodium chloride to fill many many salt shakers. Picture of formation of sodium chloride. Due to opposite charges sodium and chloride ions are held together by the electrostatic force of attraction to form sodium chloride Na Cl or NaCl. Sodium chloride crystal has a face-centered cubic close packed structureThe chloride ions o. Among many other useful sodium compounds sodium hydroxide is used in soap manufacture and sodium chloride edible salt is a de-icing agent and a nutrient for animals including humans.
Additionally this salt can be mined from the Earth.
Sodium has a configuration of 281. Therefore chlorine has aconfiguration of 287. Salt or sodium chloride is formed by neutralization of sodium hydroxide a base with hydrogen chloride an acid. Evaporation of the sea water is one of the major processes used to obtain salt and is most widely followed in countries like India. Picture of formation of sodium chloride. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function.