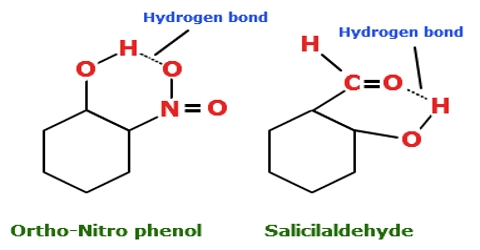
Examples Of Intermolecular And Intramolecular Hydrogen Bonding. On the basis of exhaustive searches in crystal structure databases we derive propensities for intramolecular hydrogen bond formation of five- to. H2se h2s h2po h2te chem128. Ortho - and para -nitrophenol are often used as examples of molecules with intra - and inter -molecular hydrogen bonds respectively see picture. Hence the boiling point of o nitrophenol is more than p nitophenol.

A common example of intramolecular hydrogen bonding is seen in the dimer of formic acid acetic acid o-hydroxybenzaldehyde o-nitrophenol and intermolecular hydrogen bonding in water ammonia hydrogen fluoride etc. Hydrogen bonds are actual bonds within a molecule as opposed to intermolecular forces between the separate molecules. A common example for molecules that can form intermolecular hydrogen bonds are water molecules H 2 OThe hydrogen bonds in between the water molecules cause the formation of a rigid structure when liquid water is converted into solid ice. Types of intermolecular forces all intermolecular forces are electrostatic in nature In chemistry a polar molecule is one that has uneven charge distribution. An intramolecular hydrogen bond results in the cyclization of the molecule and prevents their association. The formation of intramolecular hydrogen bonds has a very pronounced effect on molecular structure and properties.
By a students comment on item 4.
Therefore the strength of hydrogen bond in water is much greater than that compared to ammonia. Types of hydrogen bonding. Learn what intermolecular forces are understand the 3 types of intermolecular forces and get examples of each type. When a hydrogen bond forms between hydrogen and one of these three atoms hydrogen gains a partial positive charge while the electronegative atom gains a partial negative charge. Hence the boiling point of o nitrophenol is more than p nitophenol. If the hydrogen bonding takes place between two different molecules it is known as intermolecular hydrogen.