Design Of Experiments For Analytical Method Development And Validation. Agilent 1200 Series Infinity method development solution Features Details. Design of experiment DOE is based upon the principles of experimental design mathematical equations or models and outcomes of the factors. Results from method validation can be used to. DESIGN OF EXPERIMENTS Used as one of the statistical tools for validation design of experiments can help identify which factors need to be controlled in order for a system or product to pass the ruggedness test.

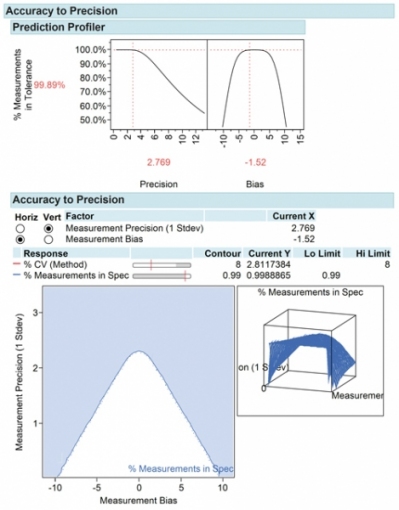
The evolution of the design of experiments to the Quality by Design paradigm for method development has been. Validation experiments to validate the developed method Stage IV. Agilent 1200 Series Infinity method development solution Features Details. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. It will be shown how DOE can be used to effectively design and improve. Analytical Methods Development Robustness of a method should be evaluated during early stages of development because results will influence the ideal technique and parameters A systematic approach should be adopted for robustness studies eg design of experiments with method parameters Start with an initial risk assessment followed with.
Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research Dehradun Uttarakhand India 1.
Elements of Validation The validation of an analytical method demonstrates the scientiic soundness of the measurement or characterization and is required throughout the. It will be shown how DOE can be used to effectively design and improve. Analytical Methods Development Robustness of a method should be evaluated during early stages of development because results will influence the ideal technique and parameters A systematic approach should be adopted for robustness studies eg design of experiments with method parameters Start with an initial risk assessment followed with. The goal is to identify the critical parameters and establish acceptance criteria for method system suitability. Choice of appropriate types of experimental designs so as to either screen the most influential factors or optimize the selected factors combination and the mathematical models in chemometry have been briefly recalled and the advantages of chemometric approaches have been emphasized. The experimental designs assist in mapping the responses on the basis of the studied objectives and exploring the critical responses at high coded as 1 medium coded as 0 or low coded as 1.