Computation And Analysis Of Protein Circular Dichroism Spectra. Introduction Circular dichroism CD is the most widely used form of chiroptical spectroscopy spectroscopic techniques that utilize the differential interaction of molecules with left and rightcircularly polarized light. Circular dichroism CD which is a consequence of molecular chirality is an important method for the investigation of protein structure and structural changes during interactions with ligands mutations and folding. PDF Circular Dichroism CD Analysis of Protein Training Poster. Analysis of circular dichroism spectra of proteins provides information about protein secondary structure.

383 2004 pp. Analytical methods developed for such an analysis use structures and spectra of a set of reference proteins. Computation and Analysis of Protein Circular Dichroism Spectra Methods Enzymol. The reference protein sets currently in use include soluble proteins with a wide range of secondary struc-. Circular dichroism measurements were recorded using a Jasco J. Methods in Enzymology 2004.
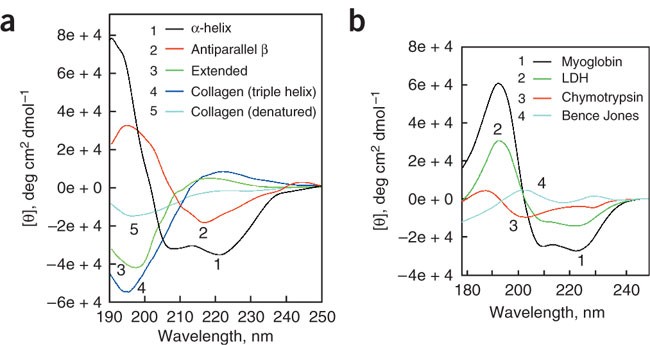
Circular dichroism CD is a valuable technique for examining the protein confor-mation in solution and assess information on the secondary and tertiary protein structure.
With the advent of synchrotron radiation circular dichroism SRCD and improvements in instrumentation for conventional CD lower wavelength data are obtainable and the information content of the spectra increased. The development of computational methods allows powerful insight to be provided into the mechanisms of generation of CD spectra in complex systems as proteins and to explain. Circular dichroism spectroscopy is a structural biology technique frequently applied to determine the secondary structure composition of soluble proteins. PDF Circular Dichroism CD Analysis of Protein Training Poster. 383 2004 pp. Circular dichroism CD which is a consequence of molecular chirality is an important method for the investigation of protein structure and structural changes during interactions with ligands mutations and folding.