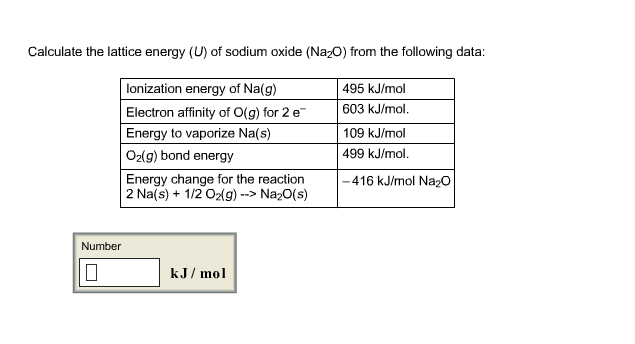
Calculate The Lattice Energy Of Sodium Oxide. O from the following data. 109 kJmol O2 g bond energy. Energy to sublime Nag 1116kJmol. 495 kJmol Electron affinity of O2 for 2e.
499 kJmol Energy change for the reaction 2Nas 12 O2g– Na2Os. Calculate the Lattice energy U of sodium oxide Na 2 Ofrom the following data. Electron affinity of Og -1225kJmol. Electron affinity of O-g 8419kJmol. 495 kJmol Electron affinity of O2 for 2e. Enthalpy change of atomisation for Nas 107kJmol1 first electron affinity of oxygen 141kJmol1 second electron affinity of oxygen 798kJmol1 enthalpy change of formation of Na 2Os 414kJmol 1.
O from the following data.
You should talk about lattice formation enthalpy if you want to talk about the amount of energy released when a lattice is formed from its scattered gaseous ions. 499 kJmol Energy change for the reaction 2Nas 12 O2g– Na2Os. The following table shows calculated values of the total lattice potential energies U pot in kJmol for crystalline salts given by H. Bond energy of O2g 4880kJmol. O 2 g bond energy. Energy to sublime Nag 1138kJmol.